FDA issues warning on several over-the-counter EYE DROPS that could cause infection and partial vision loss
11/13/2023 / By Zoey Sky
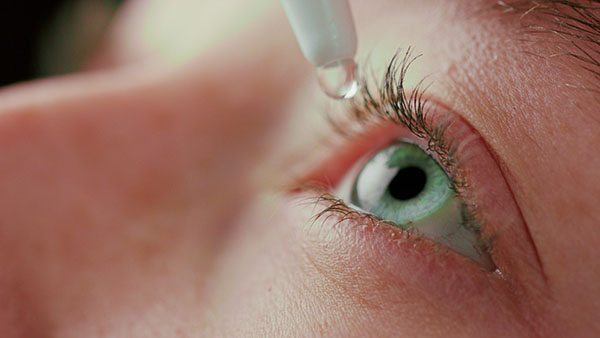
The Food and Drug Administration (FDA) has released a bulletin advising shoppers to avoid certain eye drops from several major brands because of an increased risk of developing a serious eye infection.
According to the bulletin, 26 different types of eye drops are being recalled. All of these products are sold over-the-counter (OTC).
The FDA bulletin warned that using the OTC eye drops may cause infection “that could result in partial vision loss or blindness.” The agency also advised individuals who develop symptoms of eye infection after using the products to seek medical care immediately.
According to the bulletin, the recalled eye drops are meant to be sterile. Ophthalmic drug products pose a greater risk to users because medications applied to the eyes “bypass some of the body’s natural defenses,” continued the bulletin.
The FDA recommended that manufacturers of the eye products involved recall all lots after an inspection revealed insufficient sanitary conditions in the manufacturing facility.
Additionally, FDA investigators discovered “positive bacterial test results from environmental sampling” of production areas inside the facility.
CVS, Rite Aid and Target are currently removing the products from their stores and websites. However, the agency warned that Leader, Rugby and Velocity products might still be available online and in stores and must be avoided. (Related: FDA announces voluntary recall of contaminated eye drops that could blind people.)
The FDA said it has yet to receive any adverse incident reports associated with the recalled eye drops.

According to a spokeswoman for Rite Aid, the drugstore chain is “removing the applicable Rite Aid branded products” from store shelves following the safety concerns identified by the agency. She added that Rite Aid “immediately stopped the sale in-store and online of all products supplied by Velocity Pharma within the CVS Health Brand Eye Products portfolio.”
The products listed by the FDA include:
Leader (Cardinal Health)
- Dry Eye Relief 10 ml, Polyethylene Glycol 400 0.4% & Propylene Glycol 0.3% Eye Drops
- Dry Eye Relief 15 ml, Carboxymethylcellulose Sodium Eye Drops 1% w/v
- Eye Irritation Relief 15 ml, Polyvinyl Alcohol 0.5% w/v and Povidone 0.6% w/v and Tetrahydrozoline Hydrochloride 0.05% Eye Drops
- Lubricant Eye Drops 15 ml (single pack), Carboxymethylcellulose Sodium Eye Drops 0.5% w/v
- Lubricant Eye Drops 15 ml (twin pack), Carboxymethylcellulose Sodium Eye Drops 0.5% w/v
CVS Health
- Lubricant Eye Drops 15 ml (single pack), Carboxymethylcellulose Sodium Eye Drops 0.5% w/v
- Lubricant Eye Drops 15 ml (twin pack), Carboxymethylcellulose Sodium Eye Drops 0.5% w/v
- Lubricant Gel Drops 15 ml (single pack), Carboxymethylcellulose Sodium Eye Drops 1% w/v
- Lubricant Gel Drops 15 ml (twin pack), Carboxymethylcellulose Sodium Eye Drops 1% w/v
- Lubricant Eye Drops 10 ml (single pack), Propylene Glycol Eye Drops 0.6% w/v
- Lubricant Eye Drops 10 ml (twin pack), Propylene Glycol Eye Drops 0.6% w/v
- Lubricating Gel drops 10 ml, Polyethylene Glycol 400 0.4% and Propylene Glycol 0.3% Eye Drops
- Mild Moderate Lubricating Eye Drops 15 ml (single pack), Polyethylene Glycol 400 Eye Drop ‘0.25% w/v
- Multi-Action Relief Drops 15 ml, Polyvinyl Alcohol 0.5% w/v and Povidone 0.6% w/v and Tetrahydrozoline Hydrochloride 0.05% Eye Drops
Rite Aid
- Gentle Lubricant Gel Eye Drops 15 ml, Hypromellose 0.3%, Glycerin 0.2%, Dextran 70 0.1% Eye Drops
- Lubricant Eye Drops 15 ml (twin pack), Carboxymethylcellulose Sodium Eye Drops 0.5% w/v
- Lubricant Eye Drops 10 ml (twin pack), Propylene Glycol Eye Drops 0.6% w/v
- Lubricant Gel Drops 15 ml, Carboxymethylcellulose Sodium Eye Drops 1% w/v
- Lubricating Gel Drops 10 ml, Polyethylene Glycol 400 0.4% & Propylene Glycol 0.3% Eye Drops
- Multi-Action Relief Drops 15 ml, Polyvinyl Alcohol 0.5% w/v & Povidone 0.6% w/v and Tetrahydrozoline Hydrochloride 0.05% Eye Drops
Rugby (Cardinal Health)
- Lubricating Tears Eye Drops 15 ml, Hypromellose 2910-0.3% w/v and Dextran 70- 0.1% Eye Drops
Polyvinyl Alcohol 1.4% Lubricating Eye Drops 15 ml, Polyvinyl Alcohol Eye Drops 1.4% w/v
Target Up&Up
- Up&Up Dry Eye Relief Lubricant Eye Drops 30 ml, Polyethylene Glycol 400 0.4% & Propylene Glycol 0.3% Eye Drops
- Up&Up Extreme Relief Dry Eye 15 ml (single pack), Polyethylene Glycol 400 0.4% & Propylene Glycol 0.3% Eye Drops
- Up&Up Extreme Relief Dry Eye 30 ml (twin pack), Carboxymethylcellulose Sodium Eye Drops 0.5% w/v
Velocity Pharma
- Lubricant Eye Drop 10 ml (triple pack), Propylene Glycol Eye Drops 0.6% w/v
Other recalled eye drop products in 2023
Earlier in 2023, the Centers for Disease Control and Prevention (CDC) and the FDA also issued warnings about other eye drop products, including EzriCare Artificial Tears eye drops and Delsam Pharma’s Artificial Tears, due to potential bacterial contamination that was linked to over a dozen cases of vision loss or eye removal.
Four reported deaths were linked to the earlier product recall.
The CDC issued an alert regarding an outbreak of Pseudomonas aeruginosa, a bacteria that was never reported in the U.S. prior to the outbreak. The outbreak was also linked to the use of eye drops.
According to the CDC website, the bacteria is commonly found in the environment, like in soil and water. P. aeruginosa can cause different types of infections targeting the blood, lungs or other parts of the body, particularly “after surgery.”
The agency warned that these germs continuously find new ways to avoid the effects of antibiotics used to treat the infections they cause. “Antibiotic resistance occurs when the germs no longer respond to the antibiotics designed to kill them,” warned the CDC website.
Once they develop resistance to different types of antibiotics, these germs can become multidrug-resistant.
There is no information yet if the recent FDA warning and recall of several eye drops is linked to P. aeruginosa.
Visit FDA.news for more information about other recalled products.
Watch the video below as experts expose the FDA’s vaccine plot.
This video is from The African Blogger channel on Brighteon.com.
More related stories:
SALMONELLA outbreak in various states prompts RECALL of diced onion products.
Sources include:
Submit a correction >>
Tagged Under:
big government, Big Pharma, blindness, CDC, contamination, eye drops, eye health, FDA, infections, OTC products, outbreak, pharmaceutical fraud, Product recall, product safety, products, Pseudomonas aeruginosa, vision loss
This article may contain statements that reflect the opinion of the author