Aussie woman dies after receiving first AstraZeneca vaccine dose
08/09/2021 / By Ramon Tomey
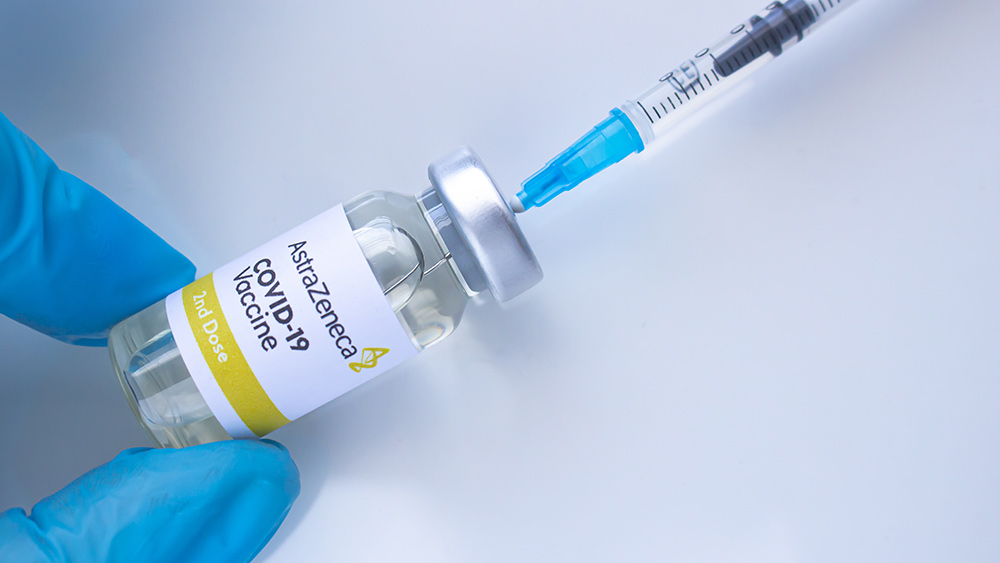
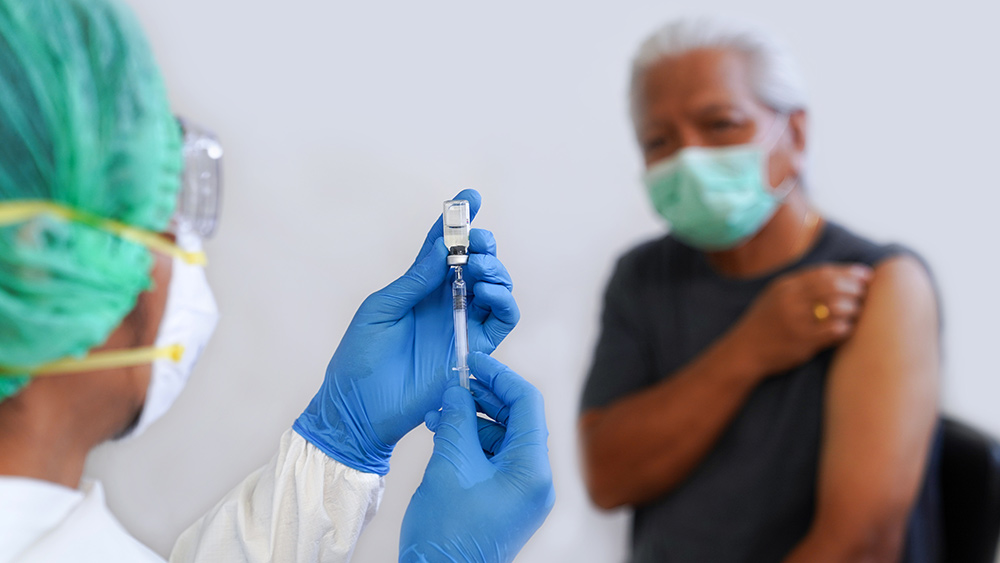
A 34-year-old woman in Australia died on Aug. 4 after she received her first Wuhan coronavirus (COVID-19) vaccine dose from AstraZeneca. Officials said the New South Wales (NSW) resident died of thrombosis with thrombocytopenia syndrome (TTS), which involved blood clots and low blood platelet count. The 34-year-old was the seventh person who died of the side effect associated with the British drug manufacturer’s COVID-19 vaccine.
The Australian Therapeutic Goods Administration (TGA) confirmed the woman’s death in the Aug. 5 edition of its weekly COVID-19 safety report. “The TGA extends its sincerest condolences to her family and loved ones. We are in close communication with [the NSW Ministry of Health], who [is] undertaking further investigation of this case,” it said.
The 34-year-old woman’s death was part of seven fatalities linked to the first dose of the AstraZeneca vaccine, made in partnership with the University of Oxford. Six deaths – including the NSW woman who died Aug. 4 – were linked to TTS. The other fatality was confirmed to be a case of immune thrombocytopenia, a disorder where the body’s immune system mistakenly attacks blood platelets responsible for clotting.
The Australian regulator said in its Aug. 5 report: “The TGA reviews all deaths reported in people who have received [COVID-19] vaccination. We also monitor the database of reports for signals that may relate to vaccine safety to distinguish between coincidental events and possible side effects of the vaccine.” Reports of suspected side effects from the AstraZeneca vaccine are “generally consistent with what is being observed internationally,” the TGA continued.
It later told the Australian Broadcasting Corporation (ABC) that severe cases of TTS commonly affected women, most especially in younger age groups. Nearly half of the women struck with TTS needed intensive care, it added. Furthermore, it found that a total of three reports of post-vaccination blood clots and low platelet count were assessed as either confirmed or probable TTS in the past week. (Related: Australian nurse hospitalized with three blood clots after receiving AstraZeneca vaccine.)
Despite the AstraZeneca vaccine’s risks, Australia still insists on using it
Leading doctors in NSW’s capital Sydney have called on people to get the AstraZeneca vaccine. The Australian Technical Advisory Group on Immunization (ATAGI) concurred with the medical professionals’ advice and defended the effectiveness of the British-made vaccine. Approximately 6.8 million doses of the vaccine have been administered in Australia as of Aug. 1, 2021.
The panel said in a statement: “ATAGI reaffirms [its] previous advice that in a large outbreak, the benefits of the COVID-19 vaccine [from] AstraZeneca are greater than the risk of … side effects for all age groups.” According to ATAGI, it issued its latest guidance amid the increasing risk of COVID-19 in Sydney and the severity of the B16172 delta strain.
While Australian authorities insisted on using the AstraZeneca vaccine, other countries have limited their use of it for certain populations – if not discontinuing it entirely. (Related: Queensland pulls AstraZeneca vaccine following more cases of blood clots.)
Two European countries – Denmark and Norway – were among the first to permanently discontinue the use of the vaccine following reports of blood clots. Søren Brostrøm, the director-general of the Danish Health Authority, announced in an April 14 statement that COVID-19 vaccinations in Denmark will move forward without the AstraZeneca vaccine.
“Based on the scientific findings, our overall assessment is [that] there is a real risk of severe side effects associated with using the COVID-19 vaccine from AstraZeneca. We have, therefore, decided to remove the vaccine from our vaccination program,” Brostrøm said.
A month later, Norway permanently suspended the use of the AstraZeneca vaccine in its COVID-19 inoculation. According to a Bloomberg report, Norwegian Prime Minister Erna Solberg announced the suspension during a May 12 news conference. “The government has decided that the AstraZeneca vaccine will not be used in Norway, not even voluntarily,” she told reporters.
Solberg’s announcement followed advice by Geir Bukholm of the Norwegian Institute of Public Health (NIPH). Bukholm, the director of the NIPH’s infection control and environmental health division, said on April 15: “We now know significantly more about the connection between the AstraZeneca vaccine and the … serious incidents of low platelets, blood clots and bleeding.”
He added: “Based on this knowledge, we have arrived at a recommendation that the AstraZeneca vaccine be removed from the [coronavirus] vaccination program in Norway.”
VaccineInjuryNews.com has more articles about the dangers of the AstraZeneca COVID-19 vaccine.
Sources include:
Tagged Under: adverse reactions, AstraZeneca, Australia, Big Pharma, Blood clots, coronavirus vaccine, covid-19 pandemic, immune thrombocytopenia, low platelet count, thrombosis with thrombocytopenia syndrome, Vaccine deaths, vaccine wars, Wuhan coronavirus